Hay’s test for bile salts:
Hay’s test for bile salts is a specific test used for the qualitative detection of bile salts in urine. Bile salt appears in the urine of patients suffering from jaundice. It consists of a watery mixture of organic and inorganic compounds. Bile acids are cholic acid and chenodeoxycholic acid which form conjugation with glycine and taurine. These two bile acids combine with sodium and potassium to form bile salts. Bile salts are sodium or potassium taurocholate, and sodium or potassium glycocholate. Hay’s test is also called sulphur powder test.
Hay’s test for bile salts principle:
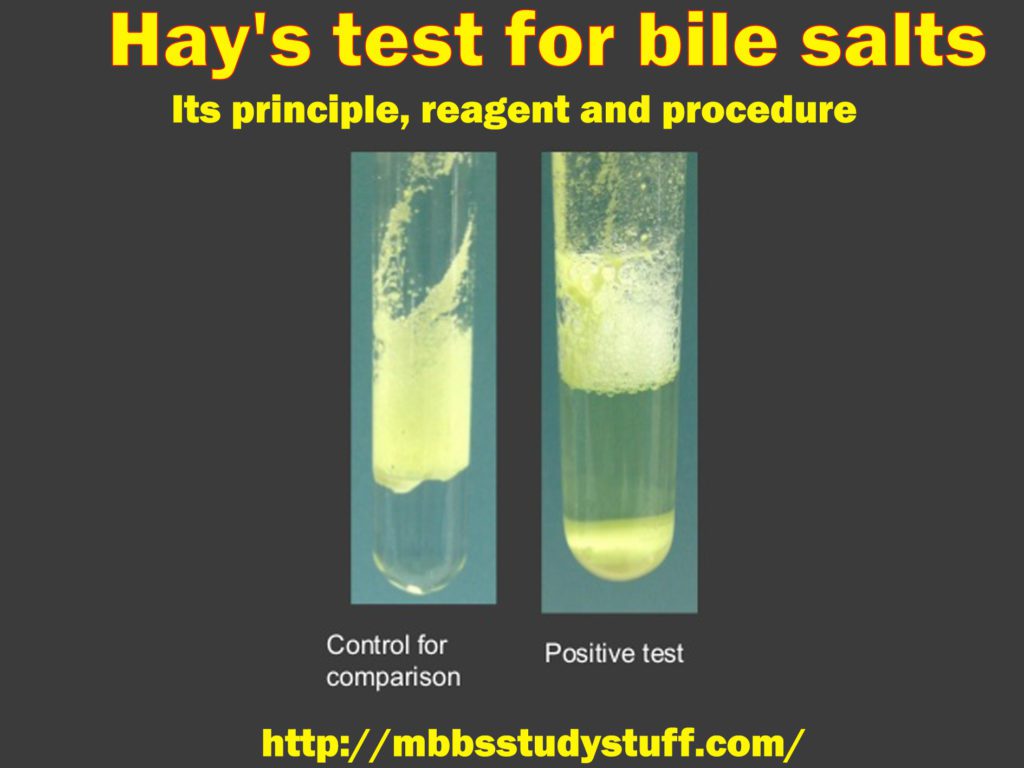
Bile salts have a property of lowering the surface tension of the fluid. If Bile salts present in urine and sulphur powder is added to the urine in the test tube, the sulphur particles will sink. In normal cases it does not sink rather, it floats on the surface of the fluids.
Hay’s test for bile salts reagent:
- Powdered sulphur.
Hay’s test for bile salts procedure:
- Take 5 ml of urine in a test tube.
- Sprinkle gently a little of finely powdered sulphur “flower of sulphur” on the surface of the urine.
Observations:
Sulphur will sink down to the bottom of the test tube, indicating the presence of bile salts in urine.
Precautions:
To obtain a better result, follow the below steps.
- Before start performing experiment make sure to wash the apparatus before and then after the experiment.
- As always, carefully handle all the chemicals in the laboratory.
- Avoid urine touching with hands while doing the experiment.
- Use test tube holder for holding test tube.
- The test tube used should be clean neatly and free of any dirt and chemicals because we will not get the proper result then, so try to use a clean and clear test tube for a correct result.
- After experiment place the apparatus in their respective place.







Why we use control in this experiment?
Low Density & Insoluble
Why do we adding sulphur only as a reagent… why don’t we use any other reagent to find out the bike salts in the urine sample
Because sulphur specific gravity is perfect for such test and is optimum according to the specific gravity of the urine