Benedict’s test for reducing sugar:
Benedict’s test for reducing sugar is a particular test for reducing substances.This test can be: used as a rough quantitative test for the clinical evaluation.
PRINCIPLE:
In the presence of reducing sugar, cuso4 gives cupric ion in an alkaline medium which is reduced to cuprous ion cu+2 to form cuprous oxide cuo2, and different coloured precipitates are formed. Sodium citrate maintains cupric ion in the solution and prevents black discolouration of cuprous oxide.
REAGENTS:
i. Copper sulphate, ii. Sodium Citrate, and iii. Sodium Carbonate. 2. Original solution (0.8.) containing a carbohydrate.
PROCEDURE:
-
- To 5ml of Benedict’s reagent in a test tube add 8 drops of sugar solution
(original solution).
- Mix thoroughly and heat to boil for 2 minutes.
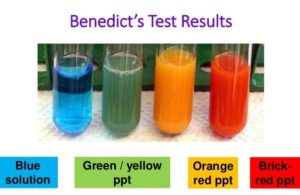
- Allow the tube to cool. The solution, in addition to the formation of a precipitate, will change colour from blue to green. Yellow, orange or red depending upon the amount of reducing sugar present.
INDICATION:
Note the colour of the precipitate at the base of the test tube.
RESULT:
The different colour precipitate formed. Green, yellow and brick red colour is formed then reducing sugar is present.
Benedict’s test for reducing sugar
Observation:
- Blue colour = no sugar or 0 % or negative
- Green colour = 0.5% sugar or +
- Yellow colour = 1% sugar or ++
- orange colour = 1.5 % sugar or +++
- Brick Red colour = 2% sugar or ++++
NOTE:
- Donot heat the test tube too much. Heat it just to boil.
Precautions:
- Wash the apparatus before and after the experiment.
- Carefully handle all the chemicals.






Leave a Reply